
Deep Dive into Propylene Glycol: What Are the Key Factors Driving Quality Differences?
1,2-Propanediol (hereafter referred to as propylene glycol unless otherwise specified) is widely used in the food, pharmaceutical, and cosmetics industries due to its lubricating, moisturizing, penetrating, and solubilizing properties. In the pharmaceutical sector, it serves as an important excipient, functioning as a solvent, humectant, stabilizer, and thickening agent.
This article will analyze the key aspects of propylene glycol products from two perspectives: production processes and impurity control.
Process Comparison — Propylene Oxide Method vs. Transesterification Method PROCESS
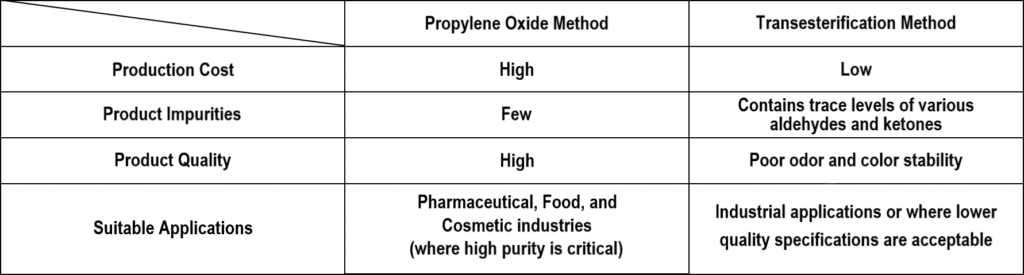
Currently, the mainstream production processes for propylene glycol include the propylene oxide method (hydration method) and the transesterification method. These two methods differ significantly in terms of process, products, and product quality.
1.1 Propylene Oxide Method
This method involves the reaction of propylene oxide with deionized water under high temperature and pressure to directly produce propylene glycol. The reaction equation is as follows:
C3H6O+H2O→C3H8O2
1.2 Transesterification Method
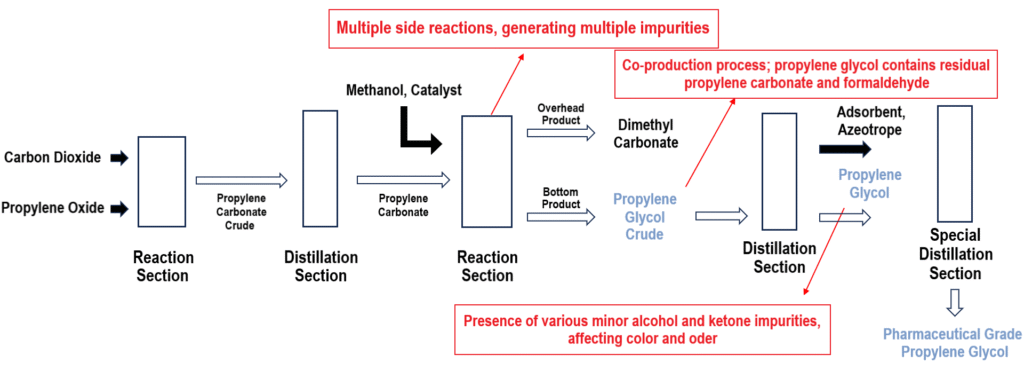
The transesterification method is currently the mainstream propylene glycol production process in China. This method primarily consists of two steps:1. Carbon dioxide reacts with propylene oxide to form propylene carbonate.
2. Propylene carbonate undergoes a transesterification reaction with methanol to produce dimethyl carbonate and propylene glycol.
The reaction equations are as follows:CH3CHCH2O+CO2→C4H6O3+CH3OH
C4H6O3+CH3OH→C3H8O2+CH3OCOOCH3
1.3 Comparison of Two Production Processes
Non-Negligible Impurity Contamination IMPURITIES
2.1 Diethylene Glycol and Ethylene Glycol Contamination — Pharmacopoeia Standards Form the Mere Baseline
On October 5, 2022, the World Health Organization (WHO) issued a medical product alert, urging regulatory authorities to recall multiple cough syrup products manufactured by India’s Maiden Pharmaceuticals. The reason was that these products contained excessive levels of diethylene glycol and ethylene glycol impurities. On April 8, 2023, the WHO issued another product alert targeting a different Indian manufacturer, again due to levels of diethylene glycol and ethylene glycol that exceeded acceptable limits. As of that time, the WHO had already issued multiple alerts concerning medicines contaminated with excessive levels of these toxic glycols.
On May 8 of the same year, the U.S. FDA released an Immediately in Effect Guidance for Industry: Testing of Glycerin, Propylene Glycol, Maltitol Solution, Hydrogenated Starch Hydrolysate, Sorbitol Solution, and Other High-Risk Drug Components for Diethylene Glycol and Ethylene Glycol.
This guidance aims to alert drug manufacturers that the reported drug components may be contaminated with diethylene glycol and ethylene glycol, posing potential health and safety risks. By the time this guidance was issued, the FDA had already received numerous reports of related poisoning cases. It is reported that between 2022 and 2023, contamination of cough syrups with diethylene glycol and ethylene glycol has caused approximately 300 child deaths worldwide.
Thus, for excipient products like glycerin and propylene glycol that are susceptible to contamination by diethylene glycol and ethylene glycol, the control and quality testing of related impurities must not be overlooked.
2.2 Impact of Aldehyde Impurities on Polypeptide Drugs
A patent titled “POLYPEPTIDE COMPOSITIONS WITH IMPROVED STABILITY” indicates that aldehyde impurities can trigger cross-linking covalent reactions in polypeptides, promoting the formation of dimers or polymers. This impact is undoubtedly detrimental to polypeptide or protein-based drugs, potentially leading to reduced efficacy or even complete loss of drug activity.
The sources of aldehyde impurities in drug components are not singular. In some cases, alcoholic impurities may also be oxidized into aldehydes. For example, studies have shown that at 400-500K (approximately 126.85°C to 226.85°C), 1,3-propanediol can react with oxygen to form acrolein, along with small amounts of formaldehyde and acetaldehyde.

It is worth noting that in pharmaceutical development and production, terminal sterilization is typically performed within the following temperature ranges:
·Moist Heat Sterilization: 115°C to 134°C (F₀ ≥ 8), with 121°C for 15 minutes being the gold standard.
·Dry Heat Sterilization: 160°C to 250°C (ranging from 2 hours to 45 minutes), where 250°C is specifically used for depyrogenation.
Therefore, for aldehyde impurities in pharmaceutical excipients, the aldehyde content alone cannot be the sole criterion; the potential impact of alcohol impurities must also be considered.
Hedinger Parenteral Grade Propylene GlycolPROPYLENE GLYCOL
The above illustrates that the quality of propylene glycol is highly dependent on the production process. For pharmaceutical/injection-grade products, impurity control is a critical consideration.
Hedinger’s high-quality parenteral grade propylene glycol is not only produced via the propylene oxide method to ensure quality and complies with multiple international pharmacopoeia standards, but it also features dedicated controls and testing for alcohol impurities such as 1,3-propanediol and 1,2-butanediol. This further reduces the risks inherent to these impurities and the potential formation of oxidative derivatives.
Furthermore, Hedinger’s propylene glycol products offer the following advantages:
·Quality control from the source: Full traceability of raw materials and their analytical data ensures strict product quality.
·Full GMP compliance: Products are packaged in cleanrooms to minimize contamination risk.
·Comprehensive batch testing: Each batch is fully tested against relevant quality standards and released only after dual verification and signing by authorized quality personnel.
·Exceptionally low endotoxin and microbial counts: Levels are significantly below the limits set in various pharmacopoeias, significantly enhancing the safety of pharmaceutical preparations.

For further information, please contact: info@gencloud-pharma.com



